The anti-leukemia 1st-class new drug developed by the team of the CPS-ZJU has been approved by the National Medical Products Administration for clinical trials.
2025-03-17 | 药学院英文网
On March 6, 2025, the chemical drug 1st class new drug TLX-83 capsule, jointly applied for by Zhejiang University, Shanghai Institute of Pharmaceutical Science of the Chinese Academy of Sciences, Yantai New Drug Creation Provincial Laboratory, and Zhongke Zhongshan Drug Innovation Research Institute, was officially approved by the National Medical Products Administration for clinical trials. It is planned to be used for the treatment of relapsed and refractory acute myeloid leukemia.
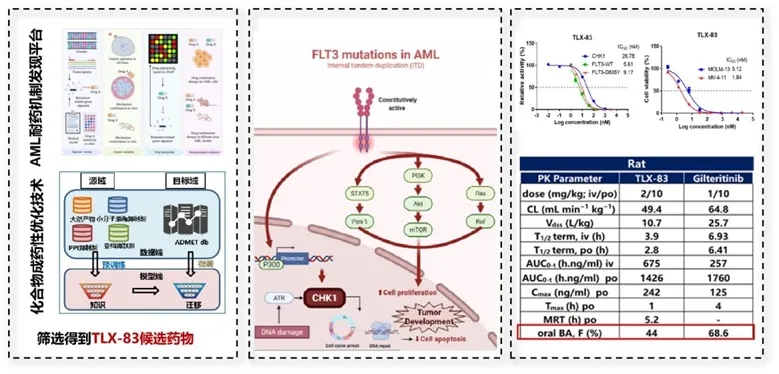
The TLX-83 is a dual-target inhibitor for FLT3/CHK1 developed by the research team led by Professors Tao Liu and Xiaowu Dong from the CPS-ZJU, and the research team from the Shanghai Institute of Pharmaceutical Science of the Chinese Academy of Sciences. Acute myeloid leukemia (AML) is an aggressive hematological tumor characterized by abnormal proliferation and differentiation of myeloid cells, with high malignancy and difficult-to-treat features. FLT3 gene mutation is a key driver factor of acute myeloid leukemia, and approximately 30% of AML patients carry such mutations. AML patients with FLT3 mutations often have poor prognosis and are prone to recurrence due to drug resistance, thus there is still an unmet clinical need for patients who are resistant to existing FLT3 inhibitors.
The research team first discovered and elucidated that the CHK1 inhibitor can overcome FLT3 inhibitor resistance by restoring or upregulating the level of p53. To address the bottleneck problem of clinical FLT3 inhibitor resistance, the research team proposed for the first time that the FLT3/CHK1 dual-target inhibitor can overcome FLT3 inhibitor resistance. Based on this, the research team adopted the strategy of target synergy verification - molecular precise design - drugability optimization to design a series of amino pyrimidine-based FLT3/CHK1 dual-target inhibitors. After multi-level screening at the molecular - cell - animal levels, TLX-83 was obtained. As a candidate compound that can overcome FLT3 inhibitor resistance, TLX-83 has excellent drugability, safety, and pharmacological characteristics. The approval of its clinical trial is expected to fill the clinical gap that existing FLT3 inhibitors cannot cover.
NEWS
-
10
2025.12
-
27
2025.11
-
25
2025.11
-
03
2025.11
-
30
2025.10
-
29
2025.10
