The team of College of Pharmaceutical Sciences, Zhejiang University develops innovative platform for long-lasting formulations: drug crystals/powder coatings
2025-02-26 | 药学院英文网
Patients with chronic diseases often face the problem of frequent use of medication over a long period of time and have poor adherence to treatment. In addition, short-acting drugs used in the treatment of chronic diseases have limitations such as large dosage fluctuations and poor therapeutic effects, which make it difficult to meet the long-term therapeutic needs of patients. Therefore, long-acting sustained and controlled release system is a key technology to improve the effectiveness and safety of clinical drug therapy for chronic diseases. Currently, long-acting drug delivery systems, such as microspheres, are able to achieve a slow release of drugs and provide a stable blood concentration, but how to further simplify the process, improve the drug loading capacity, reduce the cost and achieve precise controlled release has become a hot spot in the innovative research and development of long-acting drug formulations.
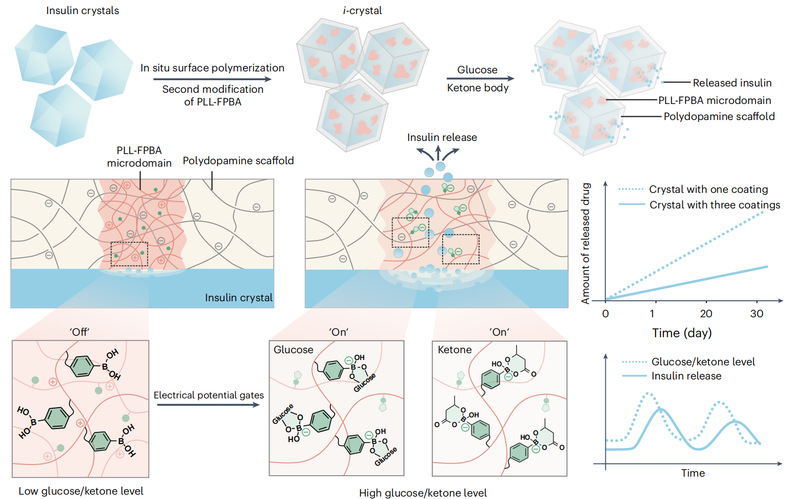
On February 26, the team of Professor Zhen Gu and Researcher Jinqiang Wang from CPS-ZJU, Jinhua Research Institute and the National Key Laboratory of Advanced Drug Delivery and Release Systems reported a new platform technology for long-lasting drug delivery: polymer cladding based on drug crystals or powders, which is capable of achieving zero-level linear slow release of drug molecules; on this basis, microdomains of pathological signaling response can be introduced into polymer membranes, which are capable of maintaining long-term stable blood drug concentration in the body according to the concentration of biomarkers of related On this basis, microdomains responsive to pathological signals can be introduced into the polymer membrane, which can precisely release drugs according to the concentration of biomarkers of relevant diseases, and maintain long-term stable blood drug concentration in the body, so as to enhance the convenience and precision of the treatment of chronic diseases. The research has been published in the journal Nature Nanotechnology, and a Research Briefing has been published to highlight the innovative drug formulation. Dr. Jianchang Xu and PhD student Yang Zhang were the first authors of this work.
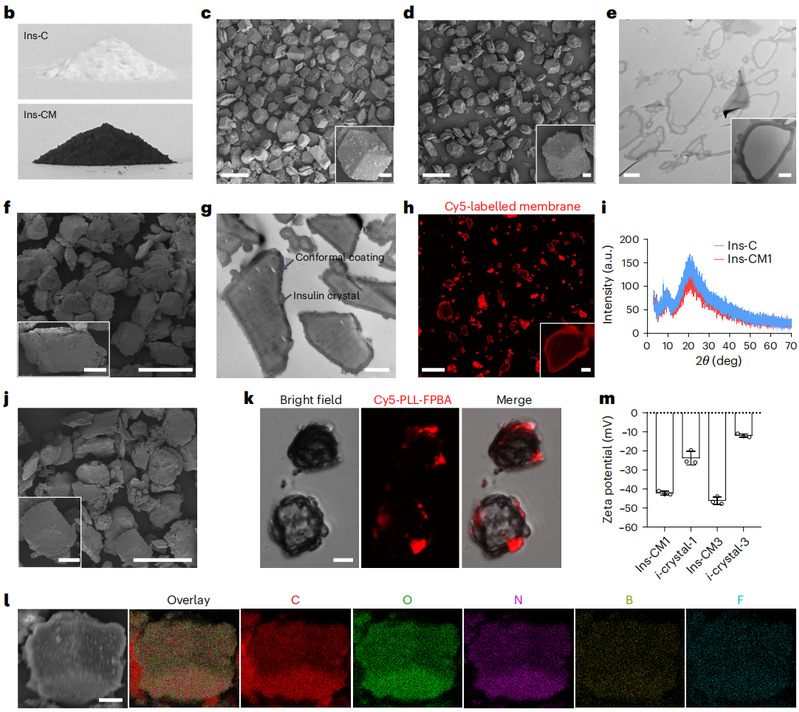
The study used insulin crystals as a model drug and type 1 diabetes as a disease model. The team polymerized in situ on the surface of insulin crystals to form a polydopamine membrane with a thickness of about 200 nm and a pore size of less than 4 nm. Due to the size limitation of the micropores, insulin was slowly released at a constant rate (zero-level release). This study is the first to achieve insulin loading higher than 90 % at a low content of carrier material (4.5 %) while being able to release insulin linearly and slowly. Subsequently, the researchers embedded PLL-FPBA in the polymer membrane to form glucose and ketone body dual-responsive microdomains to construct smart insulin crystals. After subcutaneous injection, an insulin reservoir is formed at the injection site, which releases insulin intelligently and precisely according to the blood glucose and ketone levels in the body.
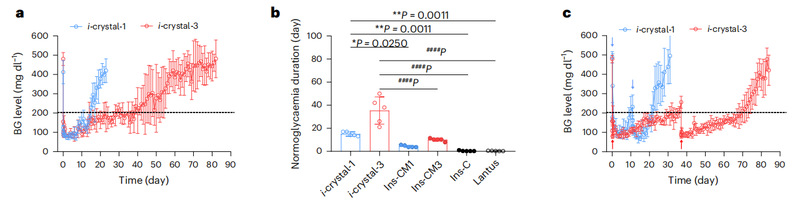
When blood glucose or blood ketones were elevated, glucose and β-hydroxybutyrate would bind specifically with FPBA in the microdomains of the polymer membrane, the membrane potential became negative, and the electrostatic repulsion between the polymer chains was enhanced, which led to an increase in the pore size of the polymer membrane and an accelerated release of insulin, thus lowering the postprandial glucose more quickly; when blood glucose or blood ketones returned to normal, the membrane potential returned to normal, and due to the size limitation of the polymer membrane micropore, insulin was released with a Zero-grade characteristics of slow release, maintaining a stable fasting blood glucose. This technology realizes for the first time the ketone body as a biomarker for insulin-responsive release. Ketone body-responsive insulin release can effectively prevent the development of ketonemia (a complication of diabetes). In a mouse model of type 1 diabetes, diabetic mice maintained normoglycemia for up to 38 days after a single administration, which was 117.2 times higher than that of commercially available long-acting glycemic insulin, and no risk of hypoglycemia was observed.
Glucose-responsive smart insulin crystal overlay for injectable formulations can enhance the duration of insulin therapy to months, which significantly improves the efficacy and safety of exogenous insulin replacement therapy and provides a new strategy for the clinical translation of long-acting insulin formulations. The drug crystal/powder coating technology can be developed into a long-acting drug delivery platform with a simple process, which can be used to coat other small molecule or biomacromolecule drugs for the treatment of different kinds of diseases by increasing the drug loading and reducing the cost at the same time.
Original link:https://www.nature.com/articles/s41565-025-01860-0
NEWS
-
10
2025.12
-
27
2025.11
-
25
2025.11
-
03
2025.11
-
30
2025.10
-
29
2025.10
