CPS-ZJU research team develops a universal chemical platform for high signal-to-background ratio near-infrared region II imaging
2024-06-26 | 药学院英文网
Optical imaging technology is widely used in the biomedical field, especially in non-invasive, real-time monitoring of processes in living organisms showing great advantages. The weakened autofluorescence and reduced absorption of organisms in the NIR-II range enable NIR-II optical imaging with higher resolution and deeper penetration depth, which is a significant advantage for imaging drug targets in deep tissues. However, the problem of the NIR-II optical probe's own background signal remains to be solved. To address this problem, activatable NIR-II probes have become a current research hotspot. However, the inherently low HOMO/LUMO energy gap of NIR-II dyes and their large π-conjugation system make it difficult to achieve the desired results by applying the traditional photophysical theory for modulation.

On 14 June 2024, Professor Xin Li's group of CPS-ZJU, published their latest research results entitled “A firm-push-to-open and light-push-to-lock strategy for a general chemical platform to develop A firm-push-to-open and light-push-to-lock strategy for a general chemical platform to develop activatable dual-modality NIR-II probes”, the authors published their latest research results in the internationally renowned journal Science Advances. In this study, the authors proposed a probe design strategy called FOLL and designed a general chemical platform for activatable dual-modality NIR-II imaging, achieving up to 50-fold signal-to-background imaging contrast in mice.
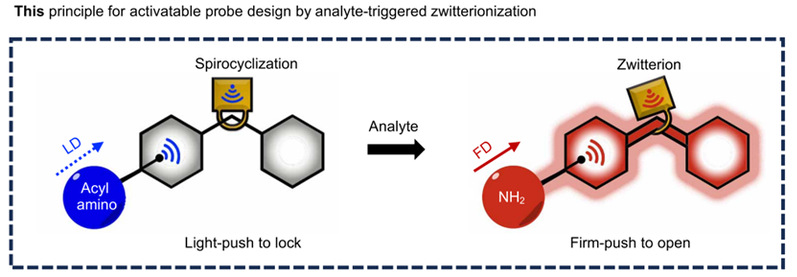
Figure 1. This principle for activatable probe design by analyte-triggered zwitterionization
Inspired by the non-fluorescent spirocyclised-strongly fluorescent amphiphile equilibrium structural properties of rhodamine derivatives, the authors proposed the design strategy of firm-push to open and light-push to lock (FOLL) by integrating the electronic effect triggered by the external target with the substituent electrophilicity inside the probe that affects the equilibrium. The authors systematically tuned the nucleophilicity of meso-positional benzene ring-neighbouring substituents in rhodamine-semilanocarbocyanine fluorophores, and designed an activatable universal chemical probe platform with high signal-to-back ratio for NIR-II imaging. Using this platform, the authors successfully designed NIR-II optical imaging probes for H2O2 and H2S, verified their practicality and versatility, and revealed the dynamics of the above two metabolites during LPS and drug-induced liver injury. This study provides an effective strategy and chemical tool for the design of NIR-II imaging probes.
The study was carried out by Professor Xin Li's group at CPS-ZJU, with first author Li Li Shen, a master's student, and Jian Li, a postdoctoral fellow, and corresponding author Professor Xin Li.
Original link:https://www.science.org/doi/10.1126/sciadv.ado2037?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
NEWS
-
10
2025.12
-
27
2025.11
-
25
2025.11
-
03
2025.11
-
30
2025.10
-
29
2025.10
