Bloodglucose-responsive oral insulin formulation developed by teams of CPS-ZJU
2024-09-04 | 药学院英文网
Diabetes affects the lives and health of over 500 million people worldwide. Of these, more than 20 percent of diabetics require exogenous insulin therapy. However, existing insulin formulations are usually administered via subcutaneous injection and absorbed into the peripheral circulation. The narrow therapeutic index of insulin leads to frequent hypoglycaemia in patients after insulin administration, which can be life-threatening in severe cases. The β-cells in healthy pancreatic can sense the blood glucose level and regulate insulin secretion precisely and intelligently. The insulin secreted by the pancreatic first enters the liver through the hepatic portal vein to play a core glucose-lowering role, and is then distributed to the periphery, enabling the body to control blood glucose levels efficiently and safely. Therefore, it is of great significance for insulin therapy to mimic the human insulin secretion mechanism, change the way of insulin administration and optimise its characteristic of peripheral effect-taking.
Recently, the team of Prof. Zhen Gu and Researcher Jinqiang Wang from College of Pharmaceutical Sciences, Zhejiang University, Jinhua Research Institute and National Key Laboratory of Advanced Drug Delivery System has developed an oral glucose-responsive insulin formulation, which can protect the loaded insulin in the gastrointestinal tract, reduce its abrupt release and degradation, and enhance its absorption, as well as form a glucose-responsive insulin reservoir in the liver to intelligently regulate the release of insulin. The formulation achieved one-day glycemic control without hypoglycemic symptoms in both mouse and pig models of type 1 diabetes. The study was recently published in the academic journal Nature Nanotechnology, with first author Kangfan Ji, a doctoral student from College of Pharmaceutical Sciences, Zhejiang University.
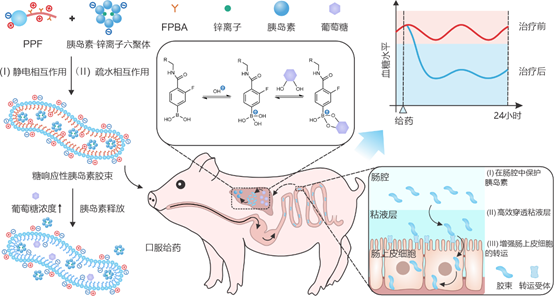
In this study, the team synthesised an amphiphilic polymer (named PPF) with an amphiphilic polybetaine at the hydrophilic end and a 4-carboxy-3-fluorophenylboronic acid (FPBA)-modified poly-2-aminoethyl methacrylate at the hydrophobic end. The micellar formulation was formed by self-assembly of PPF with insulin-zinc ion hexamer. Its unique worm-like structure, small particle size, and amphipathic ionic surface promote the efficient absorption of insulin in the gut and formation of sugar-responsive insulin reservoirs in the liver.
Under normoglycaemic conditions, the insulin in the formulation is released slowly, effectively maintaining the stability of basal blood glucose. In contrast, under hyperglycaemic conditions, glucose binds to FPBA to form a phenylborate bond, which reduces the positive charge density and hydrophobicity of the hydrophobic end of the polymer and stimulates the rapid release of insulin. The experimental results indicated that the fluorescence distribution could be clearly observed in the abdominal and liver regions of mice after gavage of fluorescently labelled micelles. The formulation first releases insulin into the liver tissue, creating a concentration gradient from the liver to the peripheral tissues that gradually decreases, thus significantly reducing the occurrence of hypoglycaemic events.
In glucose tolerance experiments in diabetic mice, glucose injection led to an increase in blood glucose in the mice in the oral formulation group and resulted in an increase in plasma insulin concentration to 2.6 times the initial value. The results of blood glucose clamp experiments in healthy and diabetic mice indicated that the rate of glucose infusion in the oral formulation group was 6% and 60% of the corresponding rate in the glucagon group, respectively. This 10-fold glucose infusion rate suggests that the mice in the oral formulation group had a higher glucose-level-dependent glucose clearance than the mice in the glycemic insulin group.
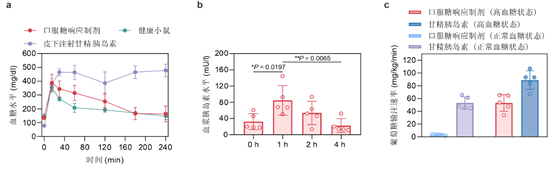
In a mouse model of spontaneous type 1 diabetes, oral administration of the dosage form for seven consecutive days was effective in controlling blood glucose in mice. In the chemically induced type 1 diabetic piglet model, once-daily feeding of micellar capsules was also effective in controlling fasting blood glucose for up to one day. Oral administration of micellar capsules maintained normoglycaemia for up to 10 hours, during which no hypoglycaemia was observed. In contrast, subcutaneous injections of glucagon caused hypoglycaemia (<50 mg/dL) in the treated mice for approximately 3.2 hours. Notably, the oral formulation reported in this article did not result in significant toxicity or proinflammatory effects in mice, and is therefore highly biocompatible.

This glucose-responsive oral insulin formulation can significantly improve the convenience, effectiveness and safety of insulin administration, and provides an innovative strategy and theoretical support for the clinical transition of oral insulin formulations.
Translator: Zihao Liu
Editor: Yichen Zhu

NEWS
-
10
2025.12
-
27
2025.11
-
25
2025.11
-
03
2025.11
-
30
2025.10
-
29
2025.10
