Research team of Zhejiang University develops a new drug delivery strategy across the blood-brain barrier
2024-05-29 | 药学院英文网
Glioblastoma can grow in areas of the brain that are inaccessible to surgery, and the probability of recurrence within 2-3 cm of the resection margin is more than 90%. The probability of recurrence of glioblastoma after standard treatment is close to 100%, in which the average survival time of patients with obvious residual lesions is only 6 months, and the survival time is even shorter when the disease recurs in the form of multiple lesions. Monoclonal antibody checkpoint inhibitors against PD-1 protein or its ligand PD-L1 have produced significant clinical efficacy in the treatment of several cancers. However, neurosystemic diseases, including glioblastoma, have been historically limited by poor penetration of blood-brain barrier drugs. The calculated molecular weight of Navumab is 146kDa, which is much larger than the 400Da allowed to pass through the blood-brain barrier, which means that antibody-mediated PD-1/PD-L1 blocking can only occur outside the blood-brain barrier.
On May 19th, Professor Hong Yuan and his team of the College of Pharmaceutical Sciences at Zhejiang University published an online research paper entitled M-MDSCs mediated trans-BBB drug delivery for suppression of glioblastoma recurrence post-standard treatment in the journal Journal of Controlled Release, which developed a novel drug delivery strategy mediated by monocyte-like myelogenic suppressor cells (M-MDSCs) across the blood-brain barrier. Professor Hong Yuan is the independent author of the paper, and Tong Yu, a doctoral student, is the only first author of the paper.

This study found that although radiotherapy and chemotherapy enhanced the expression of endothelial cell adhesion molecules associated with glioblastoma, immunosuppression-related M-MDSCs seemed to be more likely to be recruited by adhesion molecules. These M-MDSCs enter the tumor microenvironment and begin to over-express PD-L1, which helps glioblastoma deplete cytotoxic T cells, thus evading immune system killing and promoting its subsequent recurrence. In this study, a drug delivery system targeting peripheral M-MDSCs (named α Ly-6C-LAMP) was constructed, and the pcDNA3.1 vector encoding the secretory engineering trap fusion protein of PD-L1 was loaded and cloned, and M-MDSCs was used as a vector to carry drugs across the blood-brain barrier into the tumor microenvironment. As a drug delivery system easy to be prepared, intravenous injection of α-Ly-6C-LAMP can maintain a high level of PD-L1 trap protein expression in tumor-bearing mice for 3-5 days. During the treatment period, the only two administration of α-Ly-6C-LAMP significantly inhibited the intratumoral immune escape of tumor-bearing mice after routine treatment.
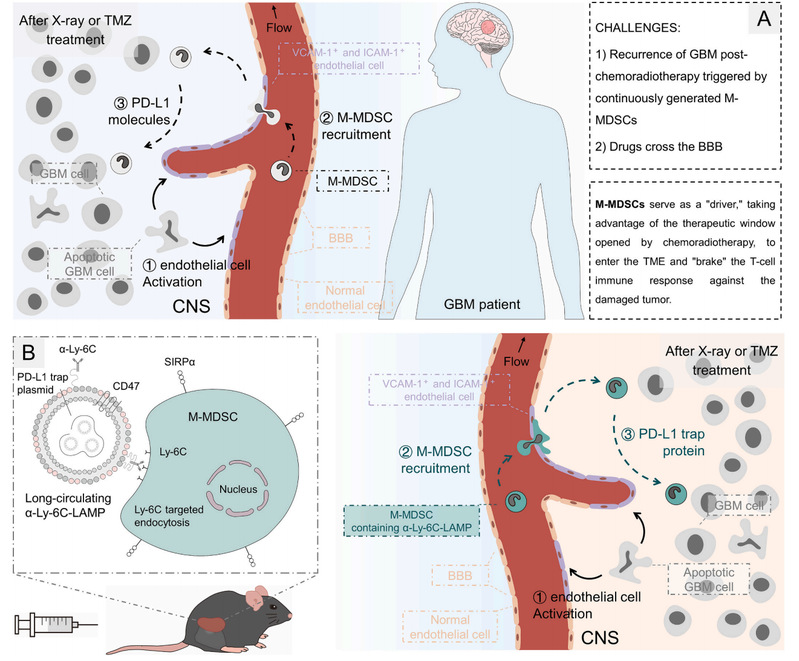
Fig. 1. A) M-MDSCs mediated GBM recurrence post-chemoradiotherapy. B) αLy-6C-LAMP targeting peripheral, especially splenic, M-MDSCs and eliciting antitumor immune responses.
This study integrates the regulation of targeted delivery and recurrence mechanism of glioblastoma, and the proposed mediating drug delivery strategy presents an efficient idea of drug delivery across the blood-brain barrier.
Link:https://www.sciencedirect.com/science/article/pii/S0168365924001962?via=ihub
NEWS
-
10
2025.12
-
27
2025.11
-
25
2025.11
-
03
2025.11
-
30
2025.10
-
29
2025.10
