Professor Jianqing Gao’s team has made progress in the field of drug/cell delivery
2022-10-12 | 药学院英文网
The team of Professor Jianqing Gao and Associate Professor Min Han, CPS-ZJU has been working on key issues in the field of drug/cell delivery. Recently, their newest research results have been published in Nano Today and ACS Nano.

The deep penetration in response to specific intratumoral conditions holds considerable potential in achieving a more effective therapy. Lactate is mainly produced by exuberant-metabolism tumor cells growing in the deep center of solid tumor, which would form the characteristic of higher-level lactate deep inside tumor in distance with blood vessels. Herein, inspired by the in vitro directional chemotaxis of enzyme-powered nanomotor driven by substrate gradient, a novel lactate oxidase (LOX)-powered Pt Nanoflowers (Pt NFs) was developed to achieve deep intratumoral penetration triggered by the lactate-driving positive chemotaxis that was confirmed both in vitro and in vivo for the first time. Besides, LOX-powered Pt NFs with self-oxygenation could achieve a more thorough effect on hypoxic relief via the cascading catalysis: (1) the orthotopic extra H2O2 production from lactate catalyzed by LOX, and (2) the effective conversion of both extra-supplement and endogenous H2O2 into O2 catalyzed by Pt NFs, further overcoming the hypoxia-induced radioresistance. Meanwhile, the X-ray radiation deposited inside Pt NFs could improve low-dose radiotherapy effect with increasing ROS level and enhancing nuclear DNA damage. Collectively, this work would provide a blueprint for developing substrate gradient-driving smart delivery systems and radiosensitization strategy.
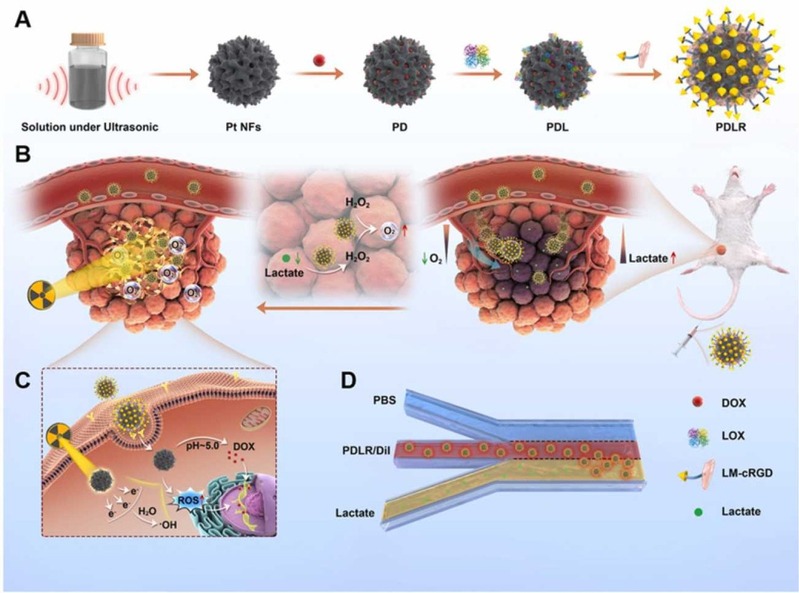
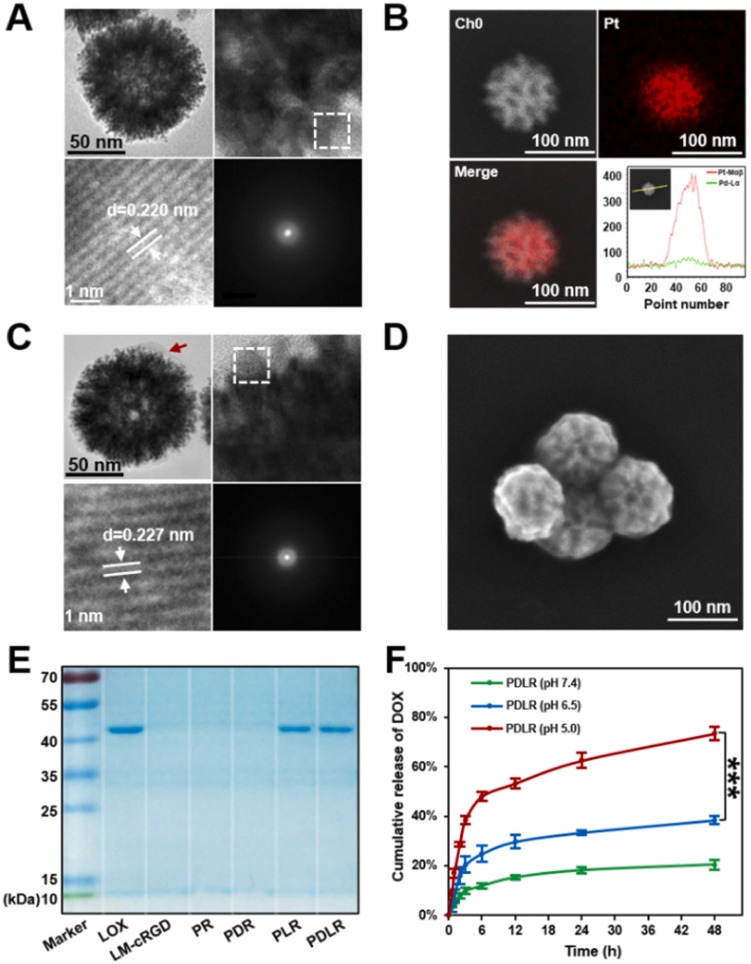

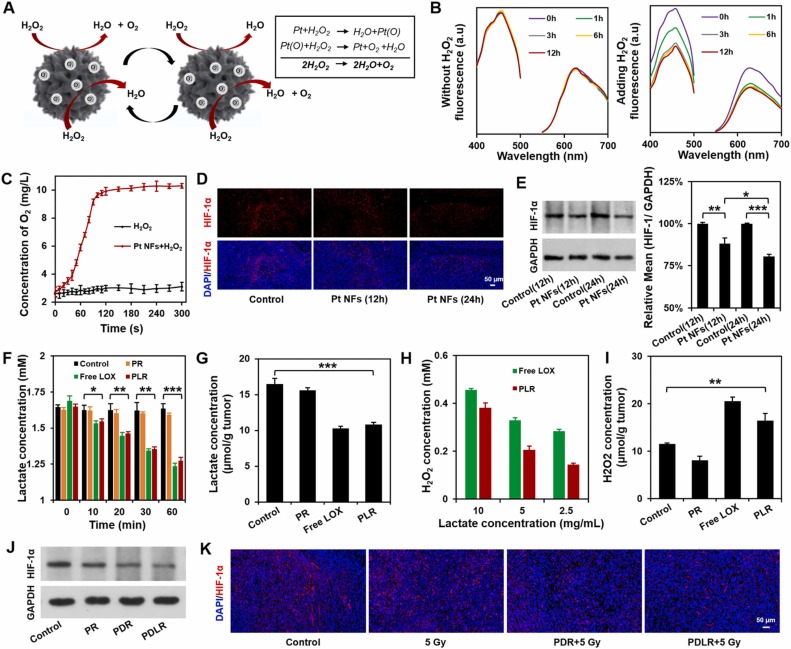
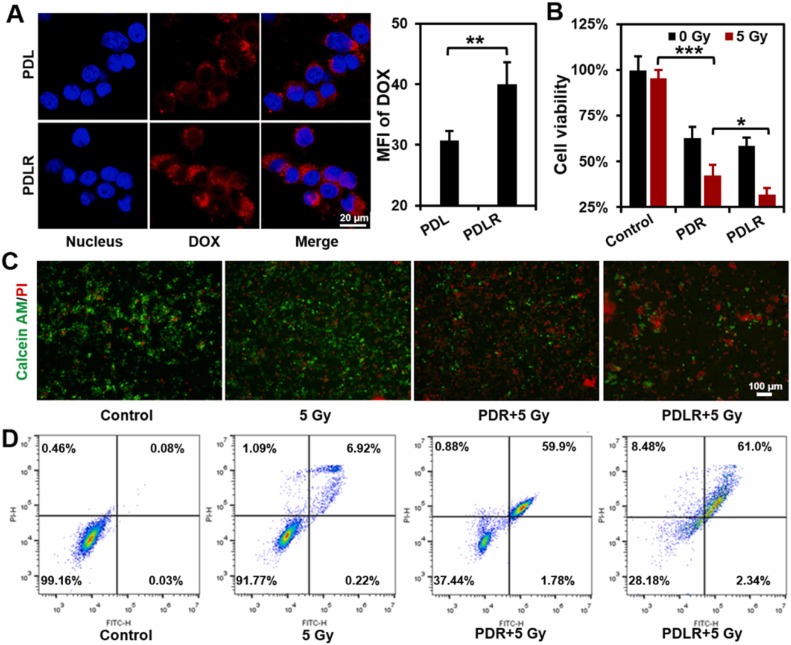
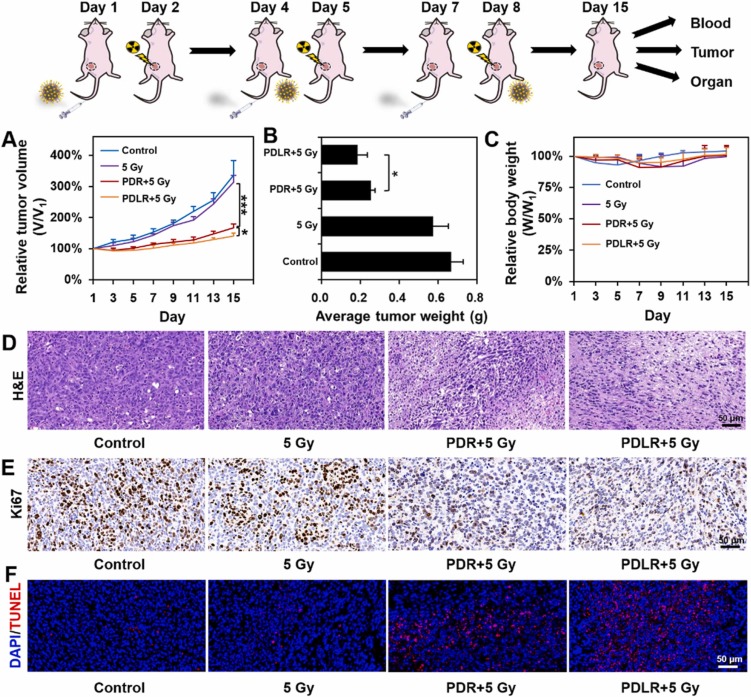
This work was carried out by Postgraduate student Zhentao Zhang and Haiqing Zhong under the supervision of Professor Jianqing Gao and Associate Professor Min Han, CPS-ZJU.
Link: https://www.sciencedirect.com/science/article/pii/S1748013222001700
The second research article entitled Stimulation by exosomes from hypoxia preconditioned human umbilical vein endothelial cells facilitates mesenchymal stem cells angiogenic function for spinal cord repai was published in ACS Nano, which focused on angiogenic nerve tissue repair strategy for spinal cord injury treatment.

Revascularization treatment is a critical measure for tissue engineering therapies like spinal cord repair. As multipotent stem cells, mesenchymal stem cells (MSCs) have proven to regulate the lesion microenvironment through feedback to the microenvironment signals. The angiogenic capacities of MSCs have been reported to be facilitated by vein endothelial cells in the niche. As emerging evidence demonstrated the roles of exosomes in cell–cell and cell–microenvironment communications, to cope with the ischemia complication for treatment of traumatic spinal cord injury, the study extracts the microenvironment factors to stimulate angiogenic MSCs through using exosomes (EX) derived from hypoxic preconditioned (HPC) human umbilical vein endothelial cells (HUVEC). The HPC treatment with a hypoxia time segment of only 15 min efficiently enhanced the function of EX in facilitating MSCs angiogenesis activity. MSCs stimulated by HPC-EX showed significant tube formation within 2 h, and the in vivo transplantation of the stimulated MSCs elicited effective nerve tissue repair after rat spinal cord transection, which could be attributed to the pro-angiogenic and anti-inflammatory impacts of the MSCs. Through the simulation of MSCs using HPC-tailored HUVEC exosomes, the results proposed an efficient angiogenic nerve tissue repair strategy for spinal cord injury treatment and could provide inspiration for therapies based on stem cells and exosomes.

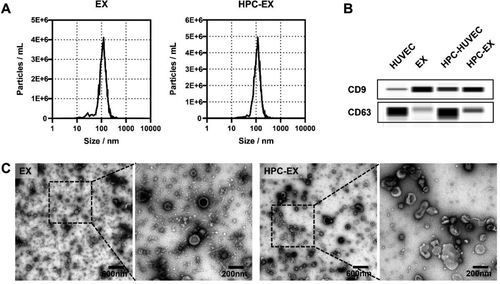

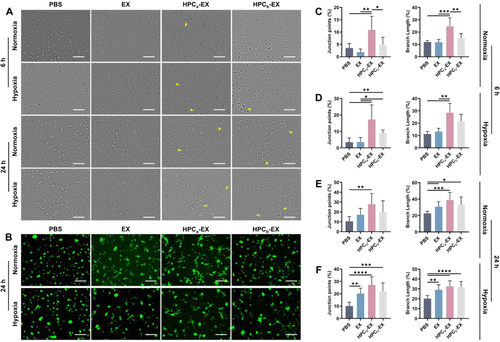
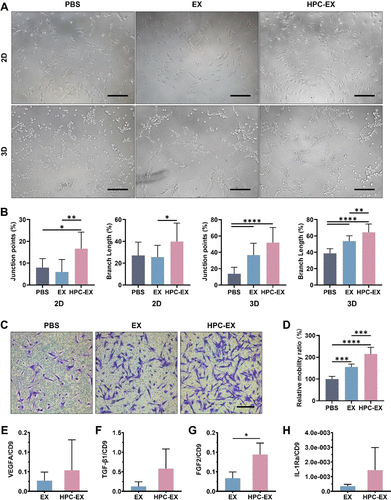
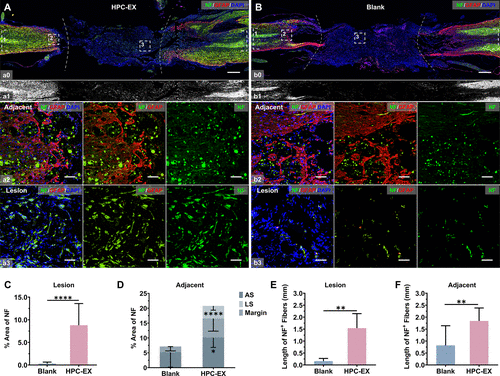
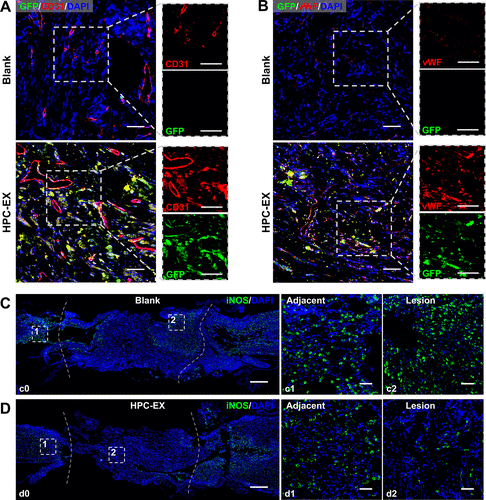
This work was carried out by postdoctor Liming Li and PhD. student Jiafu Mu under the supervision of Professor Jianqing Gao and Associate Professor Min Han, CPS-ZJU.
Link:https://pubs.acs.org/doi/10.1021/acsnano.2c02898
NEWS
-
10
2025.12
-
27
2025.11
-
25
2025.11
-
03
2025.11
-
30
2025.10
-
29
2025.10
